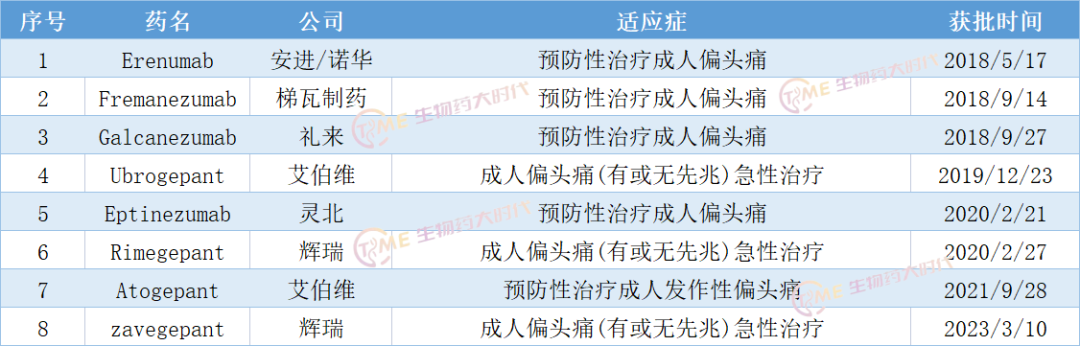
Migraine is a common neurological disorder that causes severe pain on one or both sides of the head, including nausea, vomiting, and sensitivity to light, sounds, or smells. If left untreated, a seizure can last from 4 to 72 hours and can recur, seriously affecting the patient's work and life. The pathogenesis of migraine is not well understood, but it is influenced by environmental and genetic factors and is strongly related to family history. More than 1 billion people worldwide suffer from migraines,The World Health Organization lists migraine as one of the ten most serious medical illnesses。
In practice, migraine is often treated initially with analgesics such as ibuprofen, diclofenac, and triptans, as well as antiemetics as adjunctive therapy. However, few specific drugs have been approved for the preventive treatment of migraine, so off-label drug use (OLDUs) are often used in this field, such as β blockers (commonly used to lower blood pressure), anticonvulsants (such as topiramate or sodium valproate), antidepressants (such as amitriptyline), and botulinum toxin type A.
According to the Guidelines for the Prevention and Treatment of Migraine in China (2022), nonsteroidal anti-inflammatory drugs (NSAIDs) and triptans are the main acute treatment drugs for migraine in China.
The discovery of calcitonin gene-associated peptide (CGRP), a key neuropeptide, provides new options for acute migraine/preventive treatment. At present, targeted drugs against CGRP are mainly divided into two types: CGRP monoclonal antibodies and CGRP receptor antagonists.
Rimegepant/Remegipan, acquired by Pfizer, is conducting a Phase 3 clinical trial to evaluate the efficacy and safety of Rimegepant in the prevention and treatment of migraine in Chinese subjects (registration number: CTR20230939); It has also recently been approved by the Genetic Affairs Office.
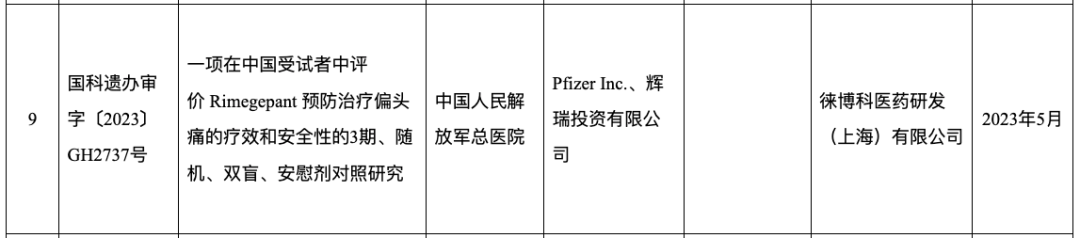
Guoke Testament Office Review [2023] GH2737: A Phase 3, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of rimegepant in the prevention and treatment of migraine in Chinese subjects; May 2023
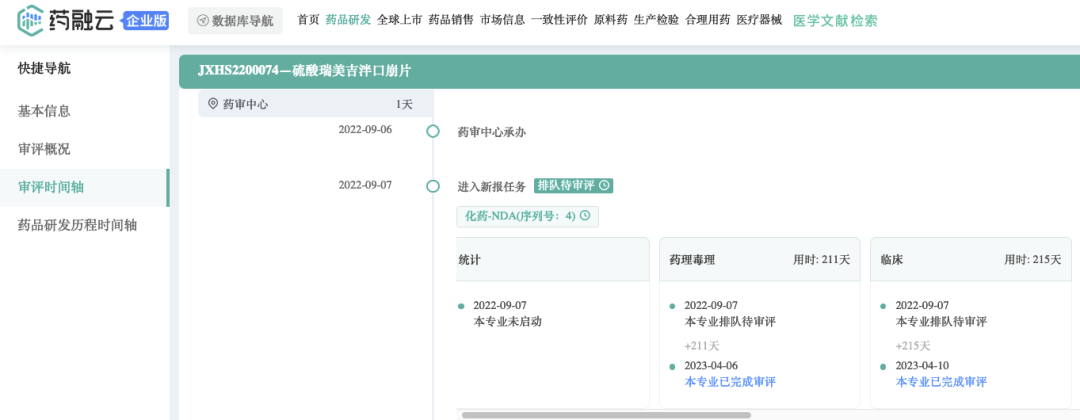
2022年9月,Rimegepant sulfate filed a new drug application (NDA) in China, and the indication of JXHS2200074 is expected to be acute migraine. It is expected that this product will be approved for marketing in China in the 3rd/4th quarter.
Previously, in November 2021, Pfizer entered into a strategic partnership with Biohaven Pharmaceutical for a total of US$1.24 billion, acquiring the rights to two CGRP antagonists and five preclinical candidate projects, including a new oral migraine small molecule drug Rimegepant(Nurtec® ODT)/This product contributed $213 million to Pfizer in 2022 (results after the completion of the acquisition in October);Global revenue reached $531 million.This product contributed $167 million to Pfizer in the first quarter of 2023.
In May 2022, Pfizer announced the acquisition of Biohaven for $11.6 billion.RimegepantOriginally discovered by Bristol-Myers Squibb (BMS).,It was later licensed to Biohaven for subsequent development.本品化合物专利WO-2011046997;It is a small molecule CGRP receptor antagonist that exerts its clinical effect by blocking the binding of CGRP ligands to receptors, and specifically treats migraine for the cause (e.g., reduction of dural artery dilation, blockade of neurogenic inflammation, inhibition of excessive pain transmission).
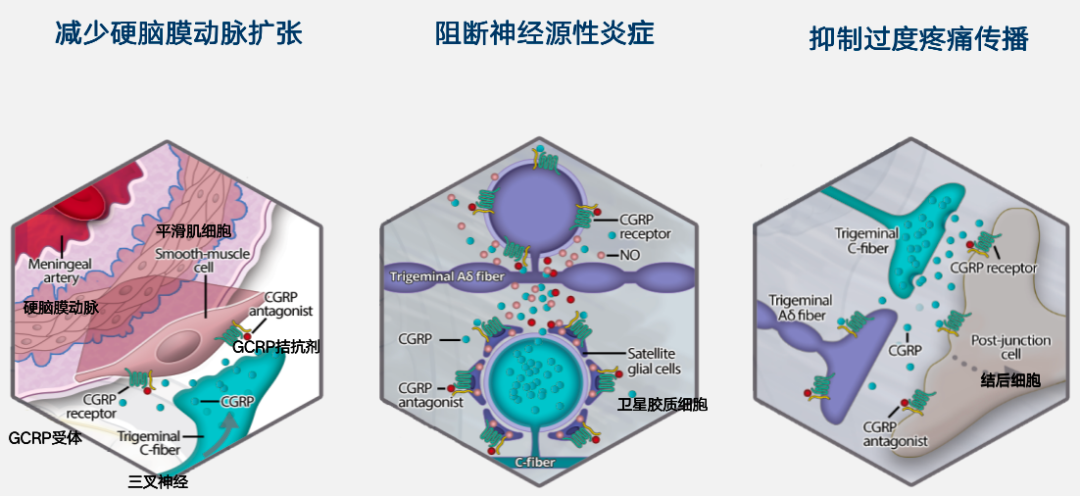
CGRP receptor antagonist mechanism
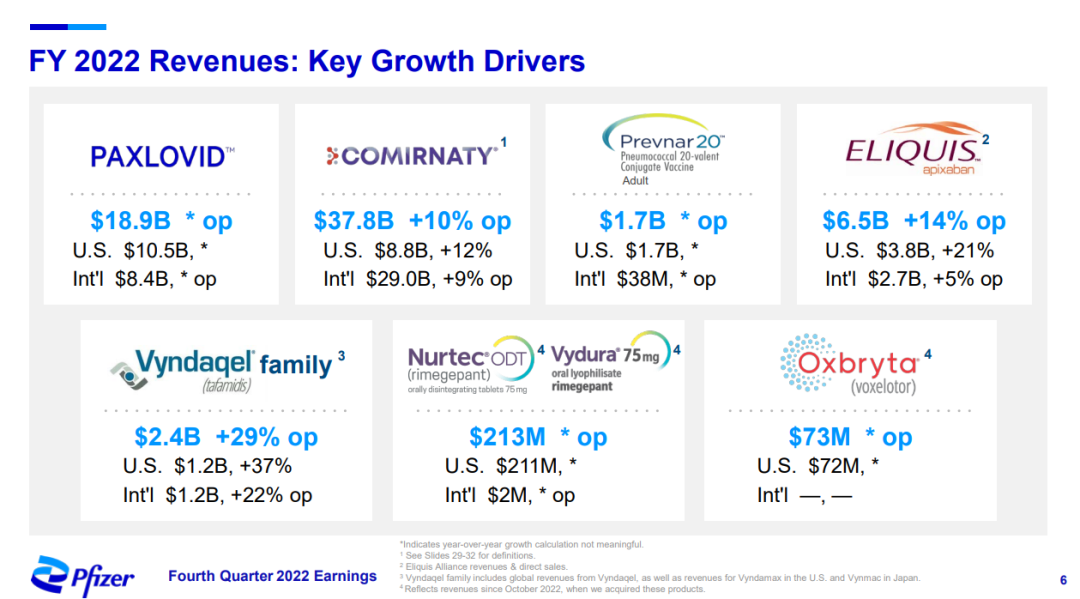
Pfizer 2022 PPT
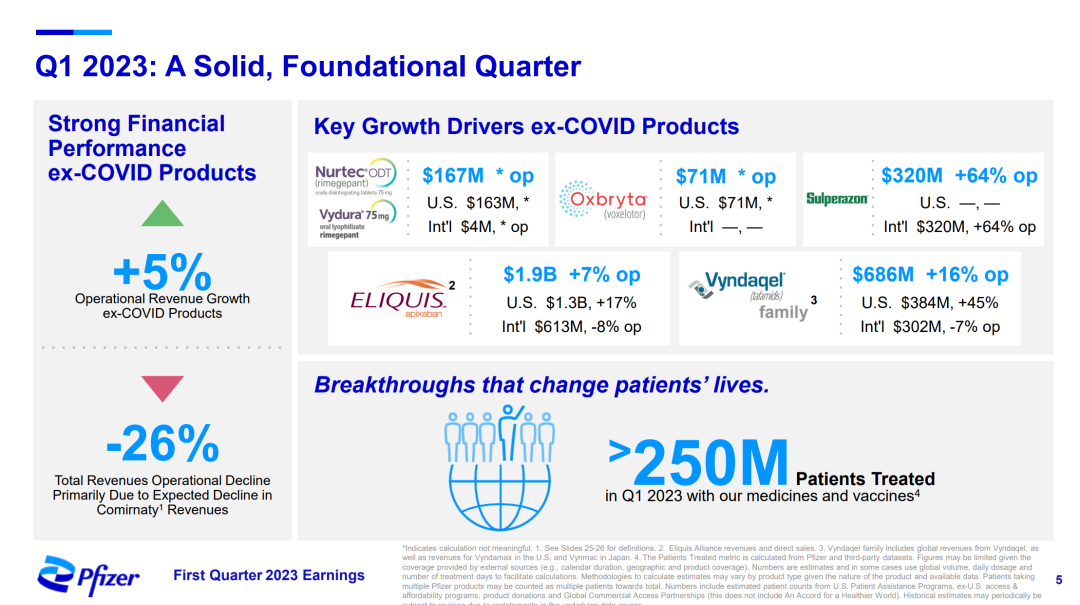
Pfizer 2023Q1 PPT
Based on the CGRP mechanism of action, Biohaven has also developed the third-generation CGRP receptor antagonist Zavegepant (nasal spray dosage form as well as gel dosage form).
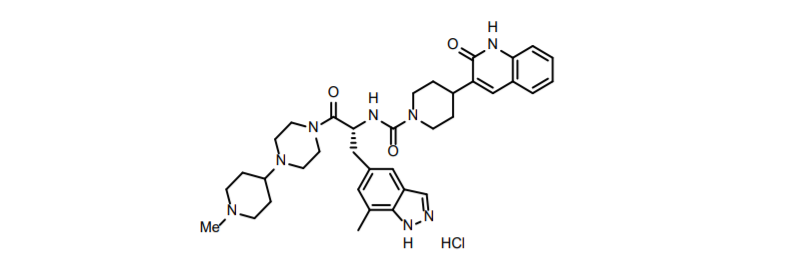
Zavegepant structure
On March 10, 2023, the US FDA approved Zavegepant (nasal spray dosage form) for the acute treatment of migraine in adults with or without aura. On May 22, 2023, China's CDE announced that Pfizer Zavegepant nasal spray has obtained implied approval for clinical trials, and intends to develop acute treatment (with or without aura) for migraine in adults.
Following the acquisition, Biohaven became a new public company focused on developing innovative therapies for neurological and other diseases. On March 22, 2023, Biohaven entered into a cooperation agreement with Hangzhou Gaoguang Pharmaceutical to introduce global rights outside Greater China of the JAK1/TYK2 inhibitor TLL-041. Biohaven made a $20 million down payment ($10 million down payment and $10 million equity investment), $950 million milestone payment and multi-tiered, proportionate sales shares.
This article is only for providing scientific information to healthcare professionals and does not represent the position of the platform
reference:
NMPA/CDE;
Pharmarong Cloud Databasewww.pharnexcloud.com;
FDA/EMA/PMDA;
Public disclosure by relevant companies;
Pfizer;
https://investors.pfizer.com/Investors/Events--Presentations/event-details/2023/Pfizer-Quarterly-Corporate-Performance--First-Quarter-2023-2023-HIzznQfeuk/default.aspx;
https://www.biohavenpharma.com/;
https://www.nurtec.com/;
https://www.bioshin.com/;

