
In March, rare disease therapies continued to be the subject of FDA attention.
Of the five approved new drugs, three were for rare diseases. Among them, Daybue even broke the gap market and became the first therapy approved by FDA for Rett syndrome.
As representatives of tumor immunotherapy, PD-1 inhibitors are also crossing over into more rare areas. Patients with Merkel cell carcinoma now have more options, and Zynyz, developed by Incyte, targeted this smaller indication to get accelerated approval from FDA, and the 5th PD-1 inhibitor in the US market was born. It is worth mentioning that Zynyz had the Greater China interest of the drug.
The star target PI3K track also welcomed a new player in March - Joenja, developed by Pharming, was approved to treat a rare primary immunodeficiency disease. However, given the unresolved safety issues of PI3K inhibitors, the market performance of Joenja may need to be seen.
In addition, for ALS, which still lacks a mitigating and curative drug, an FDA expert committee voted unanimously in March to expedite approval of Boeing/Ionis' tofersen.
1
Zavzpret: 15 minutes to migraine relief
On March 9, Zavzpret (zavegepant) received FDA approval as the first intranasal spray of a CGRP receptor antagonist for the acute treatment of adult patients with migraine.
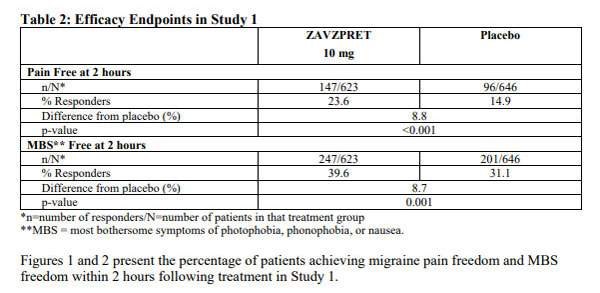
The approval is based on trial data data published in the pivotal Phase III trial of Lancet Neurology. The results showed that Zavzpret outperformed placebo on the co-primary efficacy endpoints of pain relief and MBS elimination two hours after dosing. In addition, Zavzpret was more effective than placebo on 13 of 17 secondary endpoints, including pain relief as early as 15 minutes and sustained pain relief from 2 to 48 hours post-dose.
Migraine is one of the common disabling neurological disorders, with episodes of moderately severe, throbbing headache as the main clinical manifestation. The headache is mostly lateralized, usually lasts 4-72 hours, and may be accompanied by nausea and vomiting. According to the World Health Organization, there are about 1.3 billion migraine sufferers worldwide, and the number of female sufferers is three times that of male. China is the country with the largest number of migraine sufferers, with a migraine prevalence rate of 9.3% and a population of about 130 million.
In contrast to tritans, which constrict blood vessels to stop migraines, Zavzpret aims to treat migraines at the root by blocking the calcitonin gene-related peptide receptors that cause inflammation.
The drug was acquired by Pfizer in May of last year with the purchase of Biohaven for approximately $11.6 billion in cash. As a third-generation, high-affinity, highly selective small molecule CGRP receptor antagonist, Zavzpret provides relief and prevention of migraine attacks by reversibly blocking the CGRP receptor, thereby inhibiting the biological activity of the CGRP neuropeptide. In this acquisition, Pfizer also acquired the migraine drug Nurtec ODT.
Nurtec ODT is the first oral CGRP antagonist for the prophylactic treatment of migraine and is approved in 2021. Nurtec has more than 2 million global prescriptions through 2022. Publicly available information shows that the global migraine drug market size is $6 billion in 2020, and the global drug market size in this area is expected to exceed $11 billion in 2024.
Currently, eight CGRP/CGRPR migraine drugs have been approved worldwide, namely Aimovig (Amgen/Novartis), Ajovy (Teva), Emgality (Eli Lilly), Ubrelvy (AbbVie), Vyepti (Lingbei), Vydura (Pfizer), Qulipta (AbbVie), Zavzpret (Pfizer ).
With the exception of Zavzpret, the other seven approved migraine drugs are all oral drugs. Pfizer is also developing an oral dosage form of Zavzpret.
2
Daybue: the first therapy for Rett syndrome
On March 10, the FDA approved Daybue (trofinetide) for the treatment of Rett syndrome in adults and pediatric patients two years of age and older, the first drug approved for the treatment of Rett syndrome.In 2018, Acadia entered into an exclusive license agreement with Neuren Pharmaceuticals to develop and commercialize in North America Daybue, for the treatment of Rett syndrome and other indications.
Rett syndrome is one of the most common genetic causes of severe mental retardation worldwide and is a rare condition that is X-linked dominant, occurring predominantly in females and in very rare cases in males.Rett syndrome usually starts at 6-18 months of age and presents with mental retardation, developmental delay, loss of speech and mobility, seizures, sleep disturbances, and respiratory problems. In addition to mutations in the MECP2 gene, mutations in the CDKL5 and FOXG1 genes are also responsible for the majority of cases. The pathogenic mechanism is still being investigated.
Approval of Daybue was based on data from the Phase III LAVENDER trial, which evaluated efficacy and safety compared to placebo in 187 female patients aged 5 to 20 years with Rett syndrome. Daybue demonstrated statistically significant improvement compared to placebo on both co-primary efficacy endpoints, with the most common side effects being diarrhea (82%) and vomiting (29%).
As a new synthetic analogue of the amino-terminal tripeptide of IGF-1, Daybue is designed to treat the core symptoms of Rett syndrome by potentially reducing neuroinflammation and supporting synaptic function. It was previously granted Fast Track and Orphan Drug Designation for the treatment of Rett syndrome in the U.S. and Orphan Drug Designation in Europe.
As of February of this year, there are 18 drugs in development and active for Rett syndrome, of which 4 are gene therapy drugs, 11 are chemical drugs, and 3 are of unknown type at this time.
3
Zynyz: the 5th PD-1 inhibitor in the US
On March 22, the FDA granted accelerated approval to Incyte's Zynyz (retifanlimab-dlwr) for the treatment of adult patients with metastatic or recurrent locally advanced cutaneous neuroendocrine cancer (i.e., Merkel cell carcinoma, MCC), the fifth PD-1 inhibitor approved by the FDA. Zynyz is also currently in clinical trials for the treatment of other cancer types such as non-small cell lung cancer and squamous cell carcinoma of the anal canal.
Redding Pharma entered into a development agreement with Incyte in July 2019 to acquire exclusive development rights for Zynyz in Greater China in the areas of hematology and solid tumors. However, in March this year, Redding Pharma disclosed in its 2022 financial results that it has terminated its collaboration with Incyte to develop and commercialize Zynyz in Greater China, effective January 11, 2023, based on a change in the competitive landscape.
The POD1UM-201 trial evaluated Zynyz in adults with metastatic or recurrent locally advanced MCC who had not previously received systemic therapy for advanced disease.
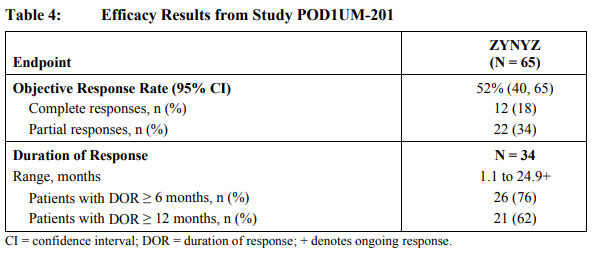
The study results showed an objective remission rate (ORR) of 52% for Zynyz monotherapy in patients not receiving chemotherapy. 76% of patients had a DOR of more than 6 months and 62% of patients had a DOR of more than 12 months. With regard to safety, 22% of patients treated with Zynyz experienced serious adverse reactions. The most common serious adverse reactions (≥2% of patients) were fatigue, arrhythmias, and pneumonia. The most common (≥10%) adverse reactions in patients treated with Zynyz were fatigue, musculoskeletal pain, pruritus, diarrhea, rash, fever, and nausea.
Merkel cell carcinoma (MCC) is a rare, highly aggressive skin cancer with neuroendocrine features that typically presents as purplish nodules in exposed areas of skin on the face, head, or neck. Its pathogenesis is unclear and the available treatments are poor, mainly the application of Mohs microsurgery for complete excision, supplemented by chemotherapy, immunotherapy, radiation therapy, and intra-lesion injection of tumor necrosis factor. These therapies have a high risk of metastatic disease and predispose to poor prognosis.
The world's first immunotherapy for metastatic Merkel cell carcinoma is Bavencio (avelumab), which received accelerated FDA approval in March 2017 and is co-developed and manufactured by Merck and Pfizer as a fully humanized IgG1 monoclonal antibody against PD-L1.
4
Rezzayo: The 10-year wait for antibacterial therapy
On March 22, Cidara and Melinta Therapeutics announced FDA approval of Rezzayo (rezafungin) for the treatment of adult patients with Candidaemia with invasive Candida who have limited or no alternative therapy. rezzayo is the first new FDA-approved echinocandin in more than a decade, Melinta said, adding that the drug will be available this summer.
Invasive candidiasis is an infection caused by a yeast (a fungus) called Candida that can affect the blood, heart, brain, eyes, bones or other parts of the body. Candidaemia is the most common form of invasive candidiasis, and more than 90% of invasive fungal infections begin in the hospital.
Previously, treatment options for the disease were limited to three antifungal drugs, only one of which was in oral form. Most therapies have become increasingly ineffective due to the increase in drug-resistant strains, and problems with toxicity, drug-drug interactions, and daily intravenous injections have made current therapies limited in many ways. Data show that despite treatment, the overall mortality rate for invasive candidiasis is still over 30%.
Unlike the three approved drugs in its class, Rezzayo does not require daily injections. As a once-a-week echinocandin, Rezzayo can be used to treat and avoid serious fungal infections such as candidemia and invasive candidiasis. The drug has been granted Fast Track status and Qualified Infectious Disease Product status by the FDA and Orphan Drug status in the U.S. and Europe for the treatment of invasive candidiasis.
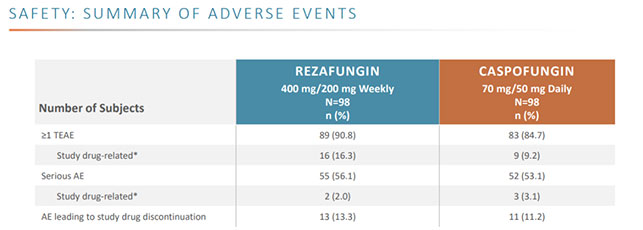
Based on the results of the global ReSTORE Phase III and STRIVE Phase II clinical trials, Rezzayo, administered once a week, achieved non-inferiority compared to caspofungin, the existing standard of care drug that must be administered daily. The most common adverse reactions to Rezzayo were hypokalemia, fever, diarrhea, anemia, vomiting, nausea, hypomagnesemia, abdominal pain, constipation, and hypophosphatemia.
In July 2022, Cidara and Melinta entered into a collaboration in which Melinta acquired an interest in the commercialization of Rezzayo in the United States in consideration for a $30 million down payment to Cidara, which also includes $60 million in regulatory milestones and up to $370 million in commercial milestone payments, tiered royalties.
Studies on the role of Rezzayo in the prevention of invasive fungal disease in adults receiving allogeneic blood and bone marrow transplants are currently underway.
5
Joenja: PI3K inhibitor new player
On March 24, Pharming announced that the FDA has approved Joenja (leniolisib) for the treatment of adult/adolescent patients 12 years of age and older with phosphatidylinositol 3-kinase δ syndrome (APDS). joenja is an oral inhibitor that selectively targets phosphatidylinositol 3-kinase δ (PI3Kδ) and is the first therapy for APDS. The therapy was originally developed by Novartis, and Pharming received development rights in 2019.
APDS is a rare primary immunodeficiency caused by mutations in the PIK3CD or PIK3R1 gene, with symptoms including severe recurrent sinopulmonary infections, lymphoproliferation, autoimmune disease and enteropathy.
The 12-week randomized controlled study showed that the study met the co-primary endpoints of lymph node reduction and correction of immunodeficiency. Compared to placebo, patients treated with Joenja had a 0.25 reduction in lymph node size index (P=0.006) and a 37.30% difference in the proportion of initial B cells (naive B cells) in peripheral blood (P=0.0002). With regard to safety, the most common adverse events in clinical trials were headache, sinusitis and atopic dermatitis.
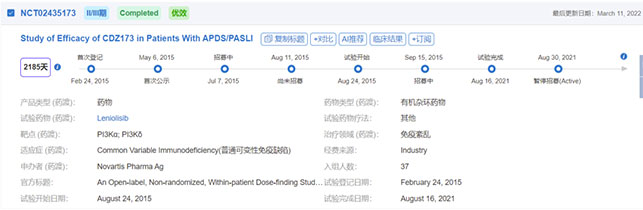
Image source: Yakult database
There are many types of PI3K inhibitors, which can be broadly classified into three major categories based on their mechanism of action: broad-spectrum PI3K inhibitors (pan-PI3K), subtype-specific PI3K inhibitors and targeted PI3K/mTOR dual inhibitors.
In addition to Joenja, a total of five PI3K inhibitors are approved worldwide, namely Gilead's Zydelig, Bayer's Aliqopa, Verastem's Copiktra, Novartis' Piqray and TG Therapeutics' Ukoniq.
PI3K has received attention from MNC as an effective target for tumor therapy. However, the development of PI3K inhibitors has suffered many setbacks due to safety concerns, and several PI3K inhibitors have suffered "misadventures" in the last year alone.
For example, Gilead withdrew its six-year PI3Kδ inhibitor IZydelig from the market; Incyte announced that it would withdraw its new drug application for PI3Kδ inhibitor Parsaclisib in the U.S.; the FDA requested MEI Pharma to conduct another trial for Zelisib; TG Therapeutics voluntarily withdrew its Ukoniq Biologic License Application/Supplemental New Drug Application for the treatment of adult patients with chronic lymphocytic leukemia and small lymphocytic lymphoma in combination with Briumvi.
PI3K is not a new target, but a perfect solution for its development in terms of drug selectivity, tumor resistance, and safety has not yet emerged, and thus more basic research is needed.
Reference:
1、FDA official website
2、Congratulations! 15 minutes to relieve migraine, the first time such therapy approved by the FDA; WuXi AppTec
3、First! FDA approves Pfizer migraine nasal spray Zavzpret (with global approval chart)
4、Peptides to attack rare diseases! The first drug DAYBUE (trefonide) for Rett syndrome was approved by FDA for marketing; Kelein Drug News
5、Merkel cell cancer new drug! FDA approves new cancer immunotherapy Zynyz (retifanlimab-dlwr); Related Jiemin Pharmaceuticals
6、10 years of waiting! These patients finally welcome the first new FDA-approved therapy; Instant Drug News
7、1 time per week! "Razafenacin" approved by the FDA for the treatment of invasive candidiasis
8、The first FDA-approved therapy for this drug-free disease; WuXi AppTec

