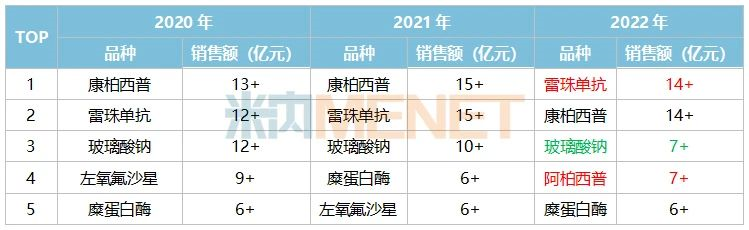
Age-related macular degeneration (AMD) is an important contributor to vision loss in elderly patients and is now the third leading cause of blindness worldwide after glaucoma and cataract.
AMD can be divided into two main types: a non-neovascularized, atrophic (dry) form and a neovascularized (wet) form; and most people with severe vision loss due to AMD have wet (wet) AMD (wAMD).
Changes in age-related macular degeneration cases
(Source: Macular Degeneration Breakthroughs)
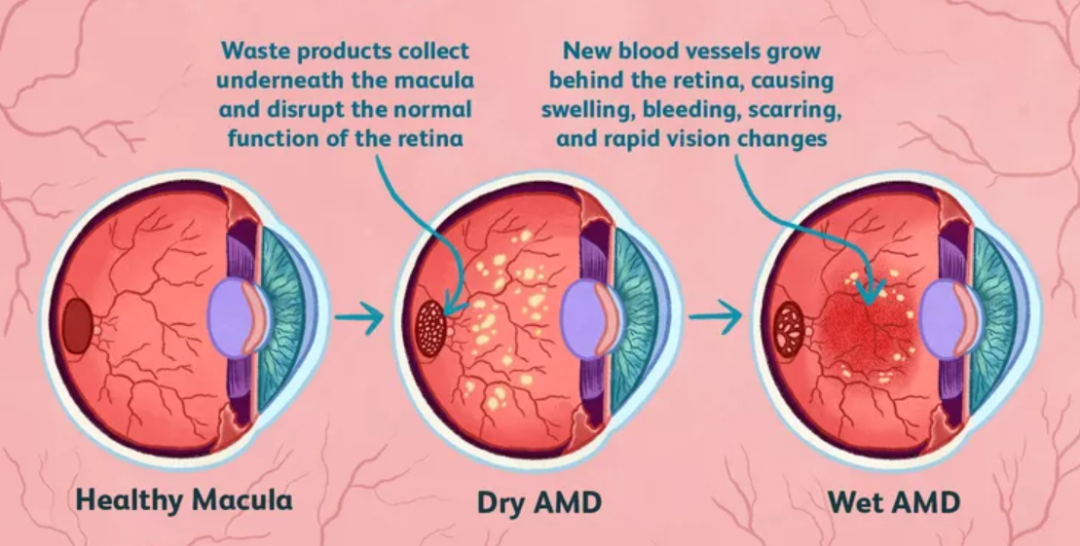
Anti-VEGF ophthalmic drugs are the standard treatment for wAMD. Four anti-VEGF ophthalmic drugs, ranibizumab, abciximab, combretastatin and brolucizumab, are currently available worldwide, of which the first three have been approved for marketing in China.
Marketed anti-VEGF ophthalmic drugs
(Source: Official website of each company, organized by Kelaiying)
Ranibizumab is the earliest anti-VEGF ophthalmic drug on the market, however, because the composition and sequence of some amino acids of the antibody produced by the hybridoma cell technology of Ranibizumab are of murine origin rather than fully humanized protein, it is easy to trigger the immune reaction with a greater chance. Moreover, Abciximab is able to bind to PlGF in addition to VEGF-A and VEGF-B, so the international rezumab market is currently being gobbled up by Abciximab step by step.
Aflibercept (Eylea) is a fusion protein anti-VEGF ophthalmic drug co-developed by Bayer and Rejuvenation, which can simultaneously block VEGF-A, VEGF-B and placental growth factor (PlGF), with a wide range of targets and long-lasting efficacy. It is mainly used for the treatment of neovascular age-related macular degeneration, retinal vein occlusion, diabetic macular edema, pathologic myopia choroidal neovascularization induced visual impairment.
It is understood that since the end of 2011, Abciximab was approved by the FDA on the market, the first year of global sales reached 838 million U.S. dollars. Subsequently, its sales have been growing at a high rate for more than ten years, and its global sales in 2021 will reach $9.4 billion, with a market share of nearly 50%, making it a real "heavy bomb". At present, abciximab is the world's highest sales volume of anti-VEGF ophthalmic drugs, its global sales in 2022 reached 9.647 billion U.S. dollars, a year-on-year growth of 4%.
And in the domestic market, Abacip's explosive power is also very strong.
MiNe data show that Abcixip was approved to enter the domestic market in February 2018, and its sales immediately exceeded 100 million yuan in 2019 after it was included in the national medical insurance class B catalog through negotiations, and its growth rate in 2020-2022 was 106.52%, 59.40% and 18.69%, respectively, and its ranking rose to TOP4 varieties in 2022, with sales of more than 700 million yuan.
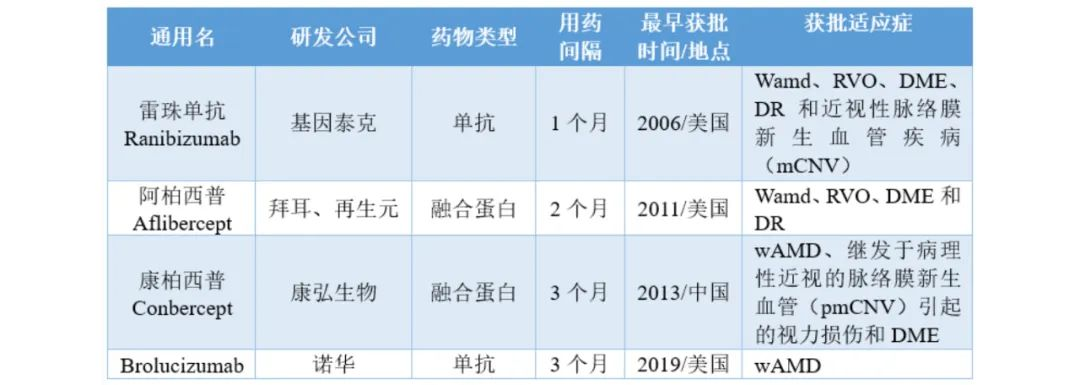
Top 5 Ophthalmic Drugs and Sales in the Last Three Years
(Source: Minnet database)
At present, there is no domestic approval of abciximab biosimilars, but there are a number of pharmaceutical companies in the layout, which Qilu Pharmaceuticals products have been reported for production, for the first domestic.2022 April CDE accepted Qilu Pharmaceuticals abciximab intravitreal injection registration application, belongs to therapeutic biologics category 3.3 drugs, the current has completed the technical review. According to the previous time of passing the registration and approval of class 3.3 biosimilars, it is expected that Qilu Pharmaceutical's Abacip intraocular injection can be launched for sale in November this year.
In addition, it is worth noting that the patent of Abciximab will expire in 2024-2025, which will greatly affect its sales performance, so the regeneration of Yuan is also expanding the indications of Abciximab in many ways. On May 11 this year, Bayer announced the initiation of a Phase III clinical study of abciximab 8mg for retinal vein occlusion, aimed at evaluating the efficacy and safety of abciximab 8mg compared to the standard treatment regimen, Eylea (abciximab 2mg), administered at extended treatment intervals in macular edema secondary to RVO.