Conference invitation letter
2023.8.3-8.4
D-G08 booth
China International Biological & Chemical Pharmaceutical Expo
【Suzhou · International Expo Center】
Dear friends & partners:
Hello everyone!
Farmasino sincerely invited everyone to participate in the "5th China International Biological & Chemical Pharmaceutical Expo 2023" on August 3-4. Gel the sense of honor and mission of the pharmaceutical man, promote the integration and innovation of pharmaceuticals, and build the prosperity and development of the pharmaceutical circle.
At this meeting, Kaiyuan Pharmaceutical has a booth (D-G08). After the Qunxian, the event will be opened, and you will invite all medicine friends to meet!
01
Overview of the meeting
The 5th CMC-China China International Biological & Chemical Pharmaceutical Expo will be held at the Suzhou International Expo Center on August 3-4, 2023! As a national professional pharmaceutical industry, the conference includes the Innovation Source Power Museum (C1), the preparation brand museum (D1), and the original auxiliary bag (E1). The conference involves cells and gene therapy, antibody drugs, small molecular innovation drugs, generic drugs, MAH B certificates, improved new drugs, ingredients, synthetic biology, green pharmaceuticals, preparation CRO, CDMO, etc. Medical experts, pharmaceutical companies, and pharmaceutical colleagues in various fields such as research, merit, and media have interested in their opinions.
02
about Us
Farmasino Co., Ltd.
Farmasino Co., Ltd. was established in Nanjing, Jiangsu in 2008. There are more than 500 employees and 7 subsidiaries. It is a comprehensive biomedical international enterprise integrating biomedical research and development, production, and trade. He is engaged in R & D business such as the consistency evaluation of generic drugs, improved new drugs and innovative drug development, CRO and MAH, and has several CDMO production bases such as pharmaceutical intermediates, raw materials, and finished medicines. The company uses industry support, develops trade, and promotes development; adheres to customers -centered, enhances corporate value -oriented, and strives to provide customers with high -quality and efficient technical support. Get a broader development space. It has a high popularity and good reputation in the field of international and domestic biomedical health.
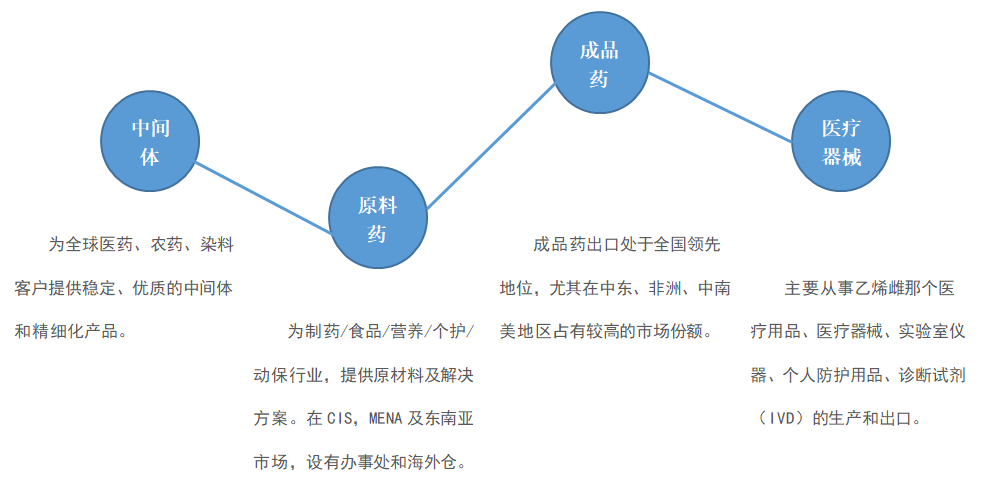
The company's main products cover pharmaceutical intermediates, raw materials, finished medicines and medical equipment, etc., products sell for more than 120 countries or regions such as the United States, Europe, Russia, India, the Middle East, Africa, Asia Pacific, and South America. Long-term relationship. The company's annual export volume exceeds 200 million US dollars, with annual sales of nearly 1.5 billion yuan. It has been awarded the "Top Ten Enterprises of West Academy Exports" by the China Medical Insurance Chamber of Commerce for 9 consecutive years. At the same time, Farmasino is also the vice president unit of the China Pharmaceutical and Health Products Import and Export Chamber of Commerce.

Jiangsu Kaiyuan Pharmaceutical Co., Ltd. is a subsidiary of Kaiyuan Pharmaceutical and is committed to the field of drug research and development. It has R & D centers covering an area of 4,000 square meters, more than 100 core technology teams, and specializes in research and development business such as chemical small molecules innovative drug development, consistency evaluation of generic drugs, CRO and MAH. Established the relationship between industry -university -research and research units such as China Pharmaceutical University and Nanjing University of Traditional Chinese Medicine and other universities and scientific research units. Strategic cooperation.
The company has obtained the "Pharmaceutical Production License" B certificate of the "MAH) holder (MAH), and has been recognized by the national" high -tech enterprise ". The company has established Jiangsu Provincial Graduate Workstation, which has obtained the national intellectual property bidding unit, Jiangsu Province's top 100 institutions, and the consistency evaluation of generic drugs Nanjing Engineering Technology Research Center, Nanjing Engineering Research Center, Nanjing Credit Manage Management Demonstration Demonstration Enterprise and other honorary titles.
01
production ability
01
Jiangsu Zhongtai Pharmaceutical Co., Ltd.
Located in the Nanjing Economic Development Zone, it is planned to build a large -scale chemical research and development center and preparation production workshop covering 20,000 square meters. The dosage types include oral solid -solid preparations, oral solutions, inhalation preparations, injections, topical drugs, and antituminants.
02
Jiangsu Beijia Pharmaceutical Co., Ltd.
The "China Medical City" located in Taizhou, Jiangsu is one of the preparation bases for Kaiyuan Pharmaceutical. The main business includes export commissioned production, MAH commissioned production, etc., and has production workshops that meet the GMP standards and hard container.
03
Kaiyuan Pharmaceutical (Anhui) Co., Ltd.
位于安徽宿州经开区,占地300亩,是开元医药生产基地之一。总投资10亿元人民币。It is located in the Economic Development Zone in Suzhou, Anhui, covering an area of 300 acres and is one of the Kaiyuan Pharmaceutical Production Base. Total investment of 1 billion yuan.
02
R & D strength
1. Expert -style R & D team
All disciplines have graduated from well -known professional colleges and have more than fifteen years of industry work experience; and hire well -known experts and scholars at home and abroad, and former supervisory institutions teachers to form a consultant team.
2. Perfect instrument and equipment
Each platform is equipped with a complete and complete instrument and equipment, covering multiple links in the research process, which can provide a good guarantee for the research and development of innovative drugs. Including quality research instruments, solid preparations, liquid preparation equipment, stability research equipment and facilities, etc.
3. Perfect quality system
Equipped with a special quality management team and quality management system to ensure the authenticity, standardization, and integrity of the test data. Passed the on -site inspection of the State Administration many times. Equipped with online version of chromatography data collection system, disaster recovery and recovery system, integrated management information system, data encryption and other intelligent information systems.
4. High -quality service guarantee
Each project is equipped with a professional project team, and the person in charge of the project conducts full -process project management to ensure the quality and efficiency of the project. Maintain timely communication with customers to ensure the smooth progress of project transfer.
Six main business
Six main business
Six superior technology platforms
1. BFS liquid preparation
2. Micron -type slow -release preparation process
3. Follow the squeezing preparation process
4. Research on Reverse Engineering in Preparation
5. Research on genetic poison/element impurities
6. Drug discovery F -ind technology platform
Corporate honor

Partner

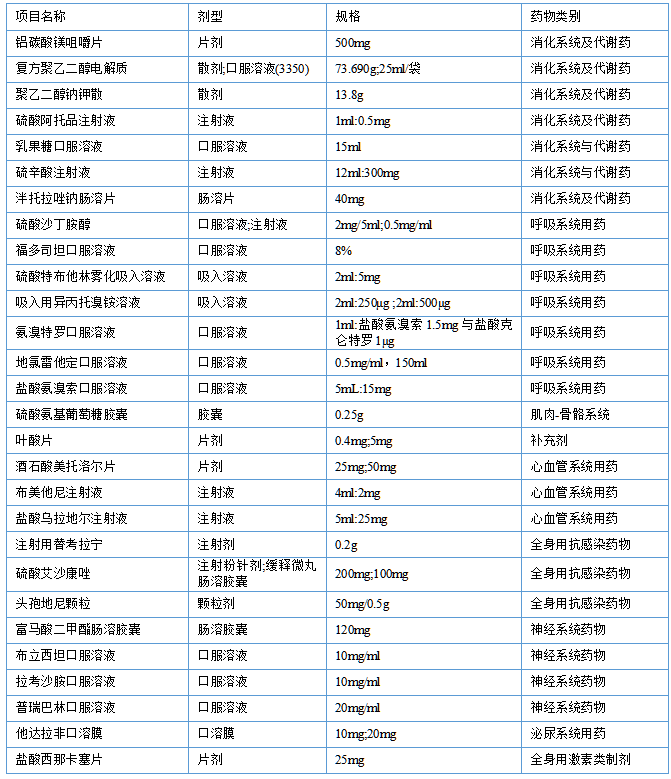
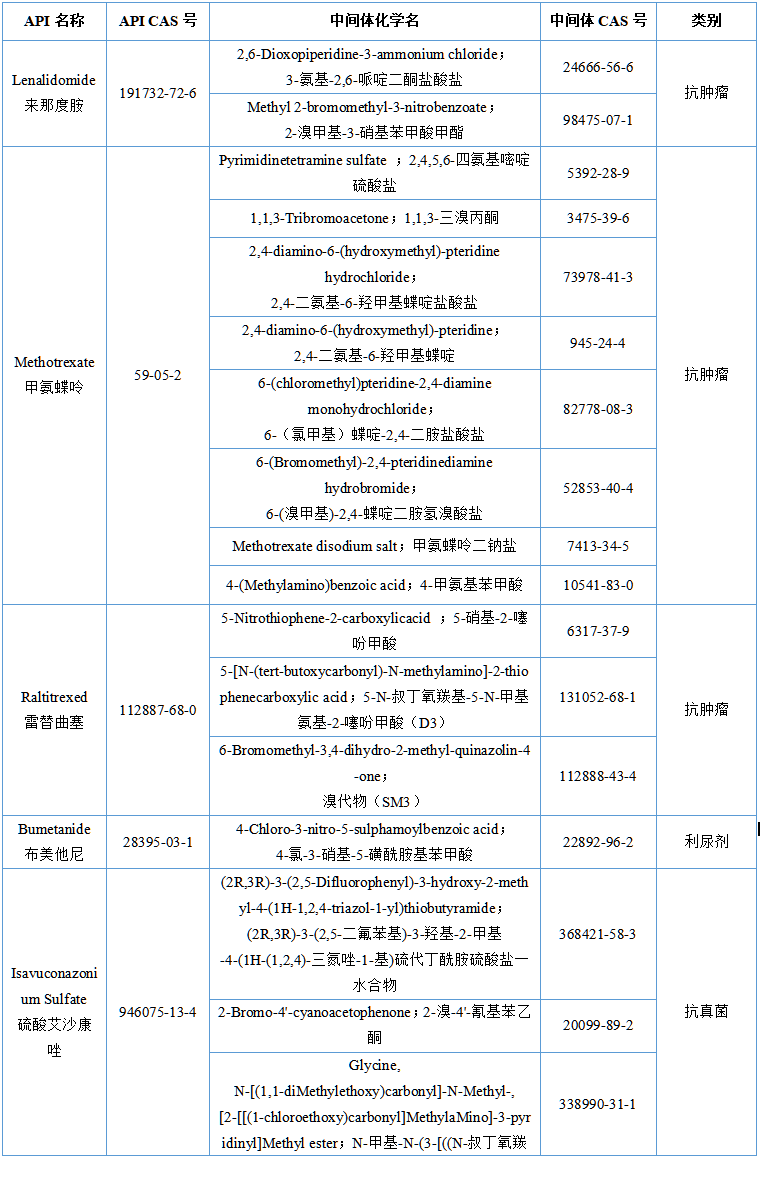
The list of items
1. Key recommendation items for preparations: